Curious (as i used to work there): Which office/code provided that grant? Do you know if it was 6.1 or 6.2 money?Hello,
We just received a large grant from the US Navy Office of Naval Research to study (and hopefully definitively answer) the question of whether oxygen is as narcotic as nitrogen, and by extension, whether there is any related advantage to nitrox. A PhD student starts work on the project next month.
Simon M
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
NITROX for any and all dives?
- Thread starter RIHappyDiver
- Start date
Please register or login
Welcome to ScubaBoard, the world's largest scuba diving community. Registration is not required to read the forums, but we encourage you to join. Joining has its benefits and enables you to participate in the discussions.
Benefits of registering include
- Ability to post and comment on topics and discussions.
- A Free photo gallery to share your dive photos with the world.
- You can make this box go away
Curious (as i used to work there): Which office/code provided that grant? Do you know if it was 6.1 or 6.2 money?
Hello Tursiops,
The university research office would understand those numbers. Unfortunately I don't (sorry). I can tell you that it was co-funded by ONR global and ONR if that makes any sense.
Other people have had a go at this previously, but what makes our project unique (and why ONR is interested in it) is that I have a global expert on the effects of anaesthetic agents on the EEG working in my department (Professor Jamie Sleigh), and he is going to help us develop an EEG-based method of objectively measuring narcosis which we hope will get around the well-known problems associated with using serial cognitive function tests in these types of investigation (such as practice effects). We will use those usual methods as well to ensure comparability with previous data, but the EEG thing is a new initiative, and could (perhaps) lead to a real time measure of narcotic impairment in divers in the future (as well as the bonus of warning of pre-seizure activity).
Simon
In
Interesting. Thanks. Good luck with it.Hello Tursiops,
The university research office would understand those numbers. Unfortunately I don't (sorry). I can tell you that it was co-funded by ONR global and ONR if that makes any sense.
Other people have had a go at this previously, but what makes our project unique (and why ONR is interested in it) is that I have a global expert on the effects of anaesthetic agents on the EEG working in my department (Professor Jamie Sleigh), and he is going to help us develop an EEG-based method of objectively measuring narcosis which we hope will get around the well-known problems associated with using serial cognitive function tests in these types of investigation (such as practice effects). We will use those usual methods as well to ensure comparability with previous data, but the EEG thing is a new initiative, and could (perhaps) lead to a real time measure of narcotic impairment in divers in the future (as well as the bonus of warning of pre-seizure activity).
Simon
No, it's not. Please pay attention. What im saying is that I've heard the argument that since O2 is metabolized in the tissues, its partial pressure there might be lower than what one would expect given the P and the FO2 in the breathing gas. And that it seems plausible. Nothing more than that.
Yeah and I am saying that I am having a problem with that argument somewhere between dissolved oxygen and oxygen bound to hemogolobin. To me it seems that one of them metabolizes while the other has "partial pressure". IANABiochemist, of course, I only work for them, so... what do I know.
Hello dmaziuk,
As a biochemist, all of this will be familiar to you, but for the benefit of others...
Oxygen bound to hemoglobin is just a way of transporting large amounts of oxygen in the blood, which is necessary because oxygen solubility in plasma is not high enough to supply tissue needs from dissolved oxygen alone.
Oxygen binding to Hb is dependent on the PO2 (the higher the PO2 the more binding of oxygen to Hb). That is why Hb carries lots of oxygen in the arterial blood where the PO2 is high (having just left the lungs). As the well oxygenated arterial blood circulates through the tissue capillaries where oxygen is being consumed, the PO2 in the blood plasma starts to fall as dissolved oxygen diffuses from blood into the tissues. As the PO2 in the blood starts to fall the Hb will unload oxygen. This release of oxygen molecules into the dissolved state has the effect of preventing the dissolved PO2 from falling as low as it would if there was no oxygen carried on Hb available, and it thus maintains the PO2 gradient for dissolved oxygen to diffuse from blood into the tissue. But importantly, the PO2 in the tissue is lower than in the blood, and the PO2 in the blood falls to lower levels than it was before the blood entered the tissue.
So Storker's point is correct. Whereas tissue pressures of nitrogen tend to equilibrate with the inspired pressure of nitrogen (quite quickly in a well perfused organ like the brain), tissue pressures of oxygen never equilibrate with the inspired pressure because of metabolism. Tissue pressures of oxygen do increase, but not to the same extent nitrogen does, and this may have an influence on the degree to which oxygen would exert a narcotic effect in comparison to nitrogen.
This effect may diminish to some extent at very high pressures of inspired PO2 where greater amounts of oxygen are dissolved in plasma; and at very high inspired PO2s the Hb may not need to unload at all and tissue pressures of oxygen may increase substantially. But those sorts of inspired PO2s are not used in diving because they are not safe.
Simon M
As a biochemist, all of this will be familiar to you, but for the benefit of others...
Oxygen bound to hemoglobin is just a way of transporting large amounts of oxygen in the blood, which is necessary because oxygen solubility in plasma is not high enough to supply tissue needs from dissolved oxygen alone.
Oxygen binding to Hb is dependent on the PO2 (the higher the PO2 the more binding of oxygen to Hb). That is why Hb carries lots of oxygen in the arterial blood where the PO2 is high (having just left the lungs). As the well oxygenated arterial blood circulates through the tissue capillaries where oxygen is being consumed, the PO2 in the blood plasma starts to fall as dissolved oxygen diffuses from blood into the tissues. As the PO2 in the blood starts to fall the Hb will unload oxygen. This release of oxygen molecules into the dissolved state has the effect of preventing the dissolved PO2 from falling as low as it would if there was no oxygen carried on Hb available, and it thus maintains the PO2 gradient for dissolved oxygen to diffuse from blood into the tissue. But importantly, the PO2 in the tissue is lower than in the blood, and the PO2 in the blood falls to lower levels than it was before the blood entered the tissue.
So Storker's point is correct. Whereas tissue pressures of nitrogen tend to equilibrate with the inspired pressure of nitrogen (quite quickly in a well perfused organ like the brain), tissue pressures of oxygen never equilibrate with the inspired pressure because of metabolism. Tissue pressures of oxygen do increase, but not to the same extent nitrogen does, and this may have an influence on the degree to which oxygen would exert a narcotic effect in comparison to nitrogen.
This effect may diminish to some extent at very high pressures of inspired PO2 where greater amounts of oxygen are dissolved in plasma; and at very high inspired PO2s the Hb may not need to unload at all and tissue pressures of oxygen may increase substantially. But those sorts of inspired PO2s are not used in diving because they are not safe.
Simon M
As a biochemist, all of this will be familiar to you, but for the benefit of others...
Thank you Simon. I am not a biochemist, I just run their computers. As a programmer I tend to notice when numbers don't add up. And that's is the one that to me doesn't:
Oxygen binding to Hb is dependent on the PO2 (the higher the PO2 the more binding of oxygen to Hb).
Each Hb molecule has N binding sites on the HEME and there's M Hb molecules in my blood stream. That puts the upper bound on O2 in my blood at N*M. According to oximeters I got hooked up to once or twice, I am normally at around 97% of that upper bound breathing 79/21% mix at 1 atm.
So I can see how I get 3 more percent at higher PO2. As I understand it, that's 3% of some 5-6% of the breathing gas I might be metabolizing.
Whereas tissue pressures of nitrogen tend to equilibrate with the inspired pressure of nitrogen (quite quickly in a well perfused organ like the brain), tissue pressures of oxygen never equilibrate with the inspired pressure because of metabolism. Tissue pressures of oxygen do increase, but not to the same extent nitrogen does, and this may have an influence on the degree to which oxygen would exert a narcotic effect in comparison to nitrogen.
I am not questioning that, I am questioning the numbers. I have really hard time seeing how a fixed upper bound of 3% of 5% turns e.g. in 20-50% differences in NDLs calculated for nitrox mixes,
To me it's much easier to believe that metabolic boost from mild hyperoxia stimulates the brain enough to better cope with narcotic effects, than to believe that O2 pressure drop causes tissue saturation so much lower that narcotic effects don't happen.
Or that O, despite being right next to N on the periodic table, does not actually bubble out of the tissue the same way N does. Or that it doesn't provoke inflammatory response in the tissues because they don't see it as foreign.
Or that Dr. Simon Mitchell says it works because empirically it appears to work.
Hello again.
I know this was a tongue-in-cheek comment but I did not say that. If you go back a couple of posts I said that we have just embarked on a substantial research project to see if oxygen is less narcotic than nitrogen. I think that is a fairly unambiguous admission that we don't know. My point was that Storker's basis for believing that oxygen might be less narcotic is valid. And it is valid.
You are misinterpreting oxygen physiology and the best path to enlightenment here may be to google some basic accounts of oxygen delivery to tissues. Maybe start with the term "oxygen cascade". You will find lots of diagrams like this one:
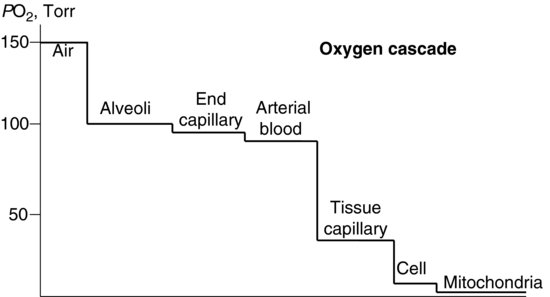
It clearly illustrates that there is a big fall in PO2 from arterial blood to cells in tissue. This still holds even when we breathe high inspired PO2s because increasing the inspired PO2 does not actually increase the carriage of oxygen on hemoglobin much (it is already 97-100% saturated with oxygen when we breathe air) and the solubility of oxygen in the plasma is low. Thus, within the range of PO2s we see in diving there will always be a gradient from arterial blood to tissues like the one you see in the diagram (in fact, the gradient gets even bigger at high PO2s - which is part of the explanation of the so-called oxygen window). In contrast, if you drew a line for nitrogen in the brain on that diagram, after a short wash-in period there would be very little difference between the nitrogen in the alveoli and the tissue nitrogen. THAT is what Storker is saying.
Simon M
dmaziuk:Or that Dr. Simon Mitchell says it works because empirically it appears to work
I know this was a tongue-in-cheek comment but I did not say that. If you go back a couple of posts I said that we have just embarked on a substantial research project to see if oxygen is less narcotic than nitrogen. I think that is a fairly unambiguous admission that we don't know. My point was that Storker's basis for believing that oxygen might be less narcotic is valid. And it is valid.
You are misinterpreting oxygen physiology and the best path to enlightenment here may be to google some basic accounts of oxygen delivery to tissues. Maybe start with the term "oxygen cascade". You will find lots of diagrams like this one:
It clearly illustrates that there is a big fall in PO2 from arterial blood to cells in tissue. This still holds even when we breathe high inspired PO2s because increasing the inspired PO2 does not actually increase the carriage of oxygen on hemoglobin much (it is already 97-100% saturated with oxygen when we breathe air) and the solubility of oxygen in the plasma is low. Thus, within the range of PO2s we see in diving there will always be a gradient from arterial blood to tissues like the one you see in the diagram (in fact, the gradient gets even bigger at high PO2s - which is part of the explanation of the so-called oxygen window). In contrast, if you drew a line for nitrogen in the brain on that diagram, after a short wash-in period there would be very little difference between the nitrogen in the alveoli and the tissue nitrogen. THAT is what Storker is saying.
Simon M
Similar threads
- Replies
- 13
- Views
- 1,018
- Replies
- 50
- Views
- 3,119
- Replies
- 2
- Views
- 607
- Question
- Replies
- 149
- Views
- 11,402




