Warning: Geeky material below!
I'm not an expert on O2 cells, but I do know a bit about analyses in general, and I've used other types of electrochemical cells in my day job. So while there probably are some flaws in my understanding, this is how I understand it.
Disclaimer: The shape of the curves I show is only for illustrative purposes. I don't know how the response curves look for a cell which has started to become current limited, so don't take the figures for more than illustrations of a principle!
An electrochemical analysis cell delivers a voltage which varies with the concentration or partial pressure of what you want to analyze. During translation from that voltage to an analysis value, there's some math involved. The response of the instrument (measured value vs real value) should be linear, but generally we can't know whether the offset is right (zero measured is zero real), nor whether the slope is correct (amount measured twice as high means that real amount is twice as high). So some instruments give you the opportunity to adjust both. In that case, we need at least two calibration points, and generally you calibrate offset at one point and slope at the other.
As a general rule, an analysis instrument should be calibrated at at least two points: One point below the expected measured value, and one point above, but that has to be weighed against the risk of a user not being properly trained messing up the calibration completely.
Recreational O2 analyzers are designed to have a constant slope and have only one adjustment, which is offset. So if your analyzer is off by 2% at 21%, it should be off by 2% at 36%. Everything is fine, no matter whether you calibrate at one point or at two points:
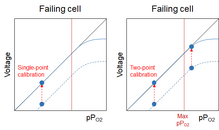
The failure mode of an O2 cell is current limitation. Which means that it starts to fail by not being able to deliver the correct current - which the instrument converts to a voltage - at the highest pPO2 (which, for us rec divers, is equal to fO2 when we analyze our tanks). So, the voltage the instrument reads is lower than it should be. As
@rjack321 said, it might still be usable if the current limitation sets in above my max fO2, but the situation might get rather nasty for a rebreather pilot who can go up to 1.6 bar pPO2 (and, AFAIK, only can go to 1.013bar during calibration). I still can't see the failure, neither with single-point nor with two-point calibration. However, with two-point calibration I can still be reasonably certain that my cell is linear in the range up to my richest mix:
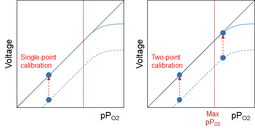
When the cell becomes even older, I might - at least theoretically - get to the point where the cell fails somewhere between 21% and my current mix. This is where two-point calibration comes in pretty handy, because it will show that I can't calibrate properly at both points. I still can't know where the failure point is, but since it's below my high calibration point I can't trust my cell anymore. If I use single-point calibration, I won't see that:
Which is why I really, really like to use two different analyzers. It isn't much of a PITA, because I always analyze twice anyway: Once during filling, with my club's analyzer, and once at the divesite, with my personal analyzer. In addition to safeguarding me a bit against a failed cell, it also allows me to check that the mix is what the label says (and I don't make the same mistake as Carlos Fonseca did...).
Only when the cell has almost completely failed will I be able to see the failure if I rely on only one analyzer and single-point calibration: