Hi guys,
I'm reading "Deco for divers", but I'm confused when reading chapters about M-value and oxygen window.
Assume that we're using air in the following questions...
1.
Everyone knows the famous "Pressure graph" that describes M-value relationship.
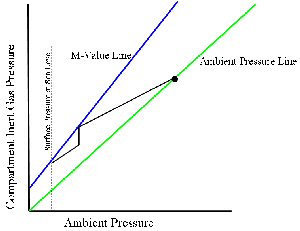
So far my understanding is that if you stay at the same depth long enough, you'll reach "Ambient pressure line" where ambient pressure equals to compartment inert gas pressure (y=x) and that's "saturation". When you start to ascend you'll go above the "ambient pressure line", and that's "supersaturation". This is confusing me, I thought if you stay at the same depth long enough, for example, at the sea level, your compartment inert gas pressure will only reach 0.79ata. And so if you're going to plot a line that indicate saturation it should be below "ambient pressure line" (i.e. y = 0.79x). Which part did I miss??
2.
According to "Deco for divers", in the chapter about oxygen window it says that off-gas speed only depends on inert gas partial pressure gradient between tissue and ambient air. However, "forming bubble" depends on the gradient between tissue total pressure (which includes O2,CO2,water,N2,...) and the ambient pressure. Once it reaches the critical point it might start to form bubbles. And the critical point is "M-value". That confused me again, if M-value depends on compartment total pressure, why do you make y-axis "compartment inert gas pressure" instead of "compartment total pressure"? What did I miss?
I'm reading "Deco for divers", but I'm confused when reading chapters about M-value and oxygen window.
Assume that we're using air in the following questions...
1.
Everyone knows the famous "Pressure graph" that describes M-value relationship.
So far my understanding is that if you stay at the same depth long enough, you'll reach "Ambient pressure line" where ambient pressure equals to compartment inert gas pressure (y=x) and that's "saturation". When you start to ascend you'll go above the "ambient pressure line", and that's "supersaturation". This is confusing me, I thought if you stay at the same depth long enough, for example, at the sea level, your compartment inert gas pressure will only reach 0.79ata. And so if you're going to plot a line that indicate saturation it should be below "ambient pressure line" (i.e. y = 0.79x). Which part did I miss??
2.
According to "Deco for divers", in the chapter about oxygen window it says that off-gas speed only depends on inert gas partial pressure gradient between tissue and ambient air. However, "forming bubble" depends on the gradient between tissue total pressure (which includes O2,CO2,water,N2,...) and the ambient pressure. Once it reaches the critical point it might start to form bubbles. And the critical point is "M-value". That confused me again, if M-value depends on compartment total pressure, why do you make y-axis "compartment inert gas pressure" instead of "compartment total pressure"? What did I miss?
Last edited:




