mlavigne
Registered
Not an actual product, and I'm not a rebreather diver yet, but thought some of you guys might find this interesting:
 www.udel.edu
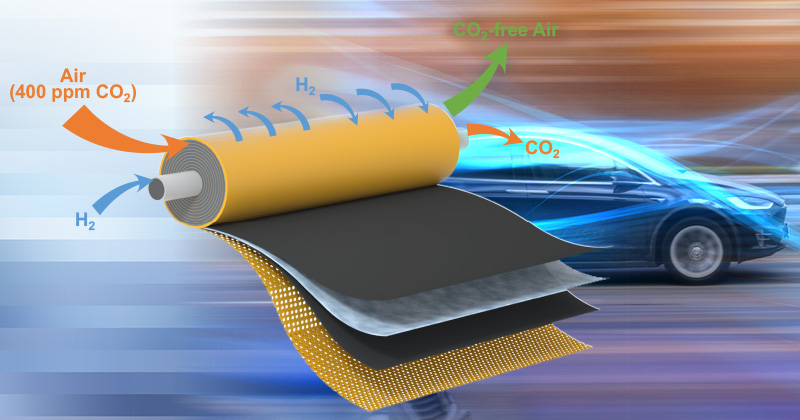
They modified a fuel cell to capture CO2 from the air (400-500ppm inlet) at 98-99% effectiveness with hydrogen gas being the only input and water + CO2 being released.
www.udel.edu
They modified a fuel cell to capture CO2 from the air (400-500ppm inlet) at 98-99% effectiveness with hydrogen gas being the only input and water + CO2 being released.
later in the article, they mention large scale rebreathers as a possible use case:
"For example, the UD-patented technology could enable lighter, more efficient carbon dioxide removal devices in spacecraft or submarines, where ongoing filtration is critical."
Based on the stochiometric ratios- you would only need 1/2 the H2 for the amount of CO2 removed, so in theory, you would never need any disposable absorbent, and would just buy H2 and O2 tanks.
If its like most research press releases, we can expect to see it available to buy somewhere between 10 year and never, but fun to think about.

Short circuit for big impact | UDaily
UD researchers report hyper-efficient method for removing carbon dioxide from air
later in the article, they mention large scale rebreathers as a possible use case:
"For example, the UD-patented technology could enable lighter, more efficient carbon dioxide removal devices in spacecraft or submarines, where ongoing filtration is critical."
Based on the stochiometric ratios- you would only need 1/2 the H2 for the amount of CO2 removed, so in theory, you would never need any disposable absorbent, and would just buy H2 and O2 tanks.
If its like most research press releases, we can expect to see it available to buy somewhere between 10 year and never, but fun to think about.




