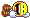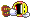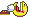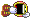.....my effort at simplyfing this....
Picture 10 blocks in a horizontal row.
These blocks represent the air, or any other gas you breathe.
For simplicity sake and to make it easier to visualize, my example won't be 100% correct in the breakdown, let's assume you are breathing 2 blocks of Oxygen and 8 Blocks of Nitrogen - right now on the surface.
Surface = 1ATA/ATM = 2 blocks Oxy, 8 blocks Nitrogen
The term Partial Pressure is the blocks. It is PART of the pressure you are breathing. Partial pressure, or the block count, changes under pressure. Easy math, every 33' of salt water is one more atmosphere.
Gas can be compressed with pressure, because of this the Partial Pressure of each part of what you breath changes.
Surface = 1ATA/ATM = 2 blocks Oxy, 8 Blocks Nitrogen
33fsw = 2ATA/ATM = 4 blocks Oxy, 16 Blocks Nitrogen
The "mix" is the same, but now the air or gas your breathing is compressed because of the pressure you're now under. So with every breath now at 33' you're breathing 4 parts Oxygen and 16 parts nitrogen.
The Total pressure reflects the sum of each Partial Pressure you breath. The total pressure at the surface is 10 based on the example I gave. At 33 feet the total pressure is 20.
As divers we have to monitor what we breath and at what depth we breath a specific mix of gas. Even air can kill you if you go too deep; Oxygen becomes toxic to us at depth if we don't pay attention to the Partial Pressure of Oxygen (part of the total pressure).
The 1.4 and 1.6 people are talking about is the partial pressure of Oxygen, just part of what you are breathing.
Mixed Gases - Nitrox, EAN2....kind of all the same thing, just a different way of saying it. Diving is about managing our gas mix (partial pressure) to extend our dive times safely.
There are really only a few ways to manage our dive times.
1) Depth - Deeper - shorter times, Shallow - longer times
2) Time - Time in the water and time out of the water
3) Changing what we breathe (higher or lower levels of Oxygen, Nitrogen or even adding a gas, say Helium)
Most divers when they use that term or phrase are only talking about Oxygen. The truth is every 'part' or 'component' of what we breath has a Partial Pressure.
PP is Partial Pressure.
PPo2 is Partial Pressure of Oxygen.
PPN is Partial pressure of nitrogen.
etc...
PT is the total pressure. P1 + P2 + P3..etc..= PT
In diving we plan our dives. We have establish the maximum time we can be in the water before we go in...or at least we should. We plan for our dive and we plan for our contingency if we go a little too deep, or stay a little too long so we don't have mandatory decompression issues.
If we use #3, and change what we breathe, then we also need to plan on what we breath becoming toxic to our bodies. That's where all the partial pressure talk leads us.
1.4 in most cases is the Partial Pressure of Oxygen for our Dive plan
1.6 in most cases is the Partial Pressure of Oxygen if we screw up or run into a problem
This is the "magic" number that tells us to get out of the water so we don't push how our body uses oxygen and prevents it from being toxic.
Hope that made it simple for you.