DandyDon,
It is my understanding that there is no carbon monoxide within the body under normal circumstances; any found there is a result of breathing air contaminated with CO, which includes most of our city air, or air behind a vehicle such as a boat or fire engine. I have measured CO levels of over 500 ppm in the eddy of a fire truck where the firemen stand--my recommendation was to keep their noses up in the air stream. CO is the result of incomplete combustion, usually from a fire or internal combustion engine.
http://en.wikipedia.org/wiki/Respiration_(physiology)
Here is an interesting PowerPoint presentation I found on sources of CO:
http://www.colorado.edu/physics/phy...ectureNotes/nagle_phys3070_fa08_lecture35.pdf
Human respiration produces CO2 exclusively, which is good as CO has a 250:1 greater association with our hemoglobin than oxygen.
Most direct-reading instruments are accurate within a certain percentage points. The Analox EII CO instrument appears to be accurate within plus or minus 30%. Detector tubes, an older CO detection technique, are plus or minus about 25%. This is not a big deal in detecting CO, but it is not a reliable method for quantitative analysis which would be required for legal purposes. Divers can use this easily to determine contamination, but this technique has limitations for accident investigation. I hope this clarifies things a bit.
John (SeaRat)
John C. Ratliff, CSP, CIH, MSPH
It is my understanding that there is no carbon monoxide within the body under normal circumstances; any found there is a result of breathing air contaminated with CO, which includes most of our city air, or air behind a vehicle such as a boat or fire engine. I have measured CO levels of over 500 ppm in the eddy of a fire truck where the firemen stand--my recommendation was to keep their noses up in the air stream. CO is the result of incomplete combustion, usually from a fire or internal combustion engine.
http://en.wikipedia.org/wiki/Respiration_(physiology)
Here is an interesting PowerPoint presentation I found on sources of CO:
http://www.colorado.edu/physics/phy...ectureNotes/nagle_phys3070_fa08_lecture35.pdf
Human respiration produces CO2 exclusively, which is good as CO has a 250:1 greater association with our hemoglobin than oxygen.
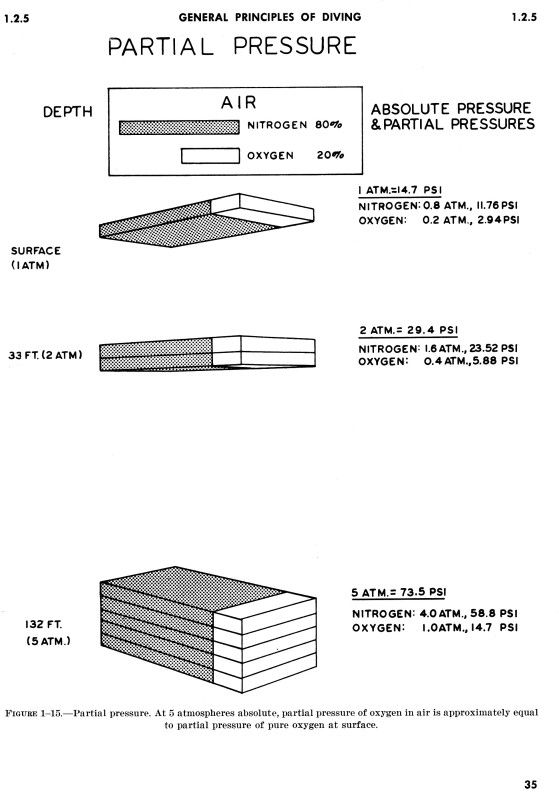
The American Conference of Governmental Industrial Hygienists sets Threshold Limit ValuesTM for substances, and for CO that level is 25 ppm. So why is it important to keep CO levels in our scuba breathing air at below 10 ppm? That is because of Dalton's Law of Partial Pressures. If we dive to 132 feet, that's five atmospheres absolute pressure, and therefore the air is compressed that we breath by a factor of five. This means that in the same space that at the surface has 10 ppm CO, at 132 feet it will have the equivalent of 50 ppm CO on the surface. At 165 feet, which we now see sport divers attaining, which is 6 atmospheres absolute, the equivalent on the surface for air actually containing 10 ppm CO would be 60 ppm CO (our bodies react to it as if there are 60 ppm in the air).Carbon monoxide combines with hemoglobin at the same point on the hemoglobin molicule as does oxygen; it can therefore displace oxygen from the hemoglobin, thereby decreasing the oxygen carrying capacity of blood. Further, it binds with about 250 times as much tenacity as oxygen, which is demonstrated bythe carbon monoxide-hemoglobin dissociation curve, except that the carbon monoxide partial pressures, shown on the abscissa, are at a level 1/250 of those for the oxygen-hemoglobin dissociation curve of Figure 40-8. Therefore, a carbon monoxide partial pressure of only 0.4 mm Hg in the alveoli, 1/250 of normal alveolar oxygen (100 mm Hg PO2), allows the carbon monoxide to compete equally with the oxygen fdor combination with the hemoglobin and causes half the hemoglobin in the blood to become bound with carbon monoxide instead of with oxygen. Therefore, a carbon monoxide pressure of only 0.6 mm Hg (a volume concentration of less than one part per thousand in air) can be lethal.
Guyton, Arthur C., M.D. and John E. Hall, Ph.D., Textbook of Medical Physiology, Eleventh Edition, Elsevier Saunders, 2006, pages 509-510.
Most direct-reading instruments are accurate within a certain percentage points. The Analox EII CO instrument appears to be accurate within plus or minus 30%. Detector tubes, an older CO detection technique, are plus or minus about 25%. This is not a big deal in detecting CO, but it is not a reliable method for quantitative analysis which would be required for legal purposes. Divers can use this easily to determine contamination, but this technique has limitations for accident investigation. I hope this clarifies things a bit.
John (SeaRat)
John C. Ratliff, CSP, CIH, MSPH
Last edited: