Hate to be the bearer of bad news, but Boyles Law, Henrys Law and Daltons law are all directly related.
noooo... volume has nothing to do with henry's law, ZERO!
here is yet another site explaining henry's law... note that volume is not talked about once, because volume is irrelevant... henry's law works because there is an increase in pressure, but has ZERO to do with volume...
Solubility of gases in liquids, Henry's law
Now, wanna talk about related laws, then we can talk about Boyles, Charles, Gay-Lussac's laws, which together make up the combined gas laws. Add Avogadros and we have the ideal gas law.
Charles Law = volume and temperature
Boyles law = volume and pressure
Gay-Lussac = pressure and temperature
Avogadros = deals with the above three and makes some statements about the ideal gas law
then we have henry's law... henry's law = the absorption of molecules from a gas into a liquid because of partial pressures
here's why it doesn't deal with the above three...
I'll use the picture from the link above...
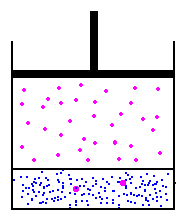
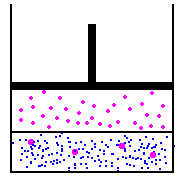
in this image, we have a fixed amount of liquid at the bottom. on the left, we'll assume that the gas and the liquid are in equilibrium. On the right, we could use Boyles law to increase the pressure by reducing the volume - this would then cause more molecules to start being absorbed into the liquid at the bottom, because the new pressure of the molecules in the gas is higher, the partial pressure is higher. To figure this out, we would use Henry's law to determine how much would be absorbed into the liquid. Funny thing about Henry's law though, is that it doesn't matter the volume - there is no place to input volume on the calculation... So, lets theorize one other option... lets add pressure, but not reduce the volume... This is simple to do, and in effect, every breath we take on scuba, we are doing this - the volume of our lungs does not change a whole lot as we dive - we increase the pressure to prevent this... so, lets add air to the container above with an air compressor... volume has not changed, but the pressure is higher, so, does Boyles law matter? Absolutely not... because we have increased the pressure without changing the volume by adding more molecules to the container, we have increased the pressure without worrying about the volume... this causes henry's law to take action, and again, because the partial pressure of the gas is higher than the partial pressure of the gas in the liquid, the liquid will start absorbing the gas...
forgot you mentioned daltons law... it is related to henry's law, and essentially just says that the sum of all partial pressures in a gas equal the total pressure of the gas... that is the basis for calculating the differences between standard air diving and mixed gas diving... boyles law still doesn't come in to play, because volume, again, is irrelevant...






