Russoft
Contributor
This whole thing can be explained rationally with science.
See this here phase diagram?
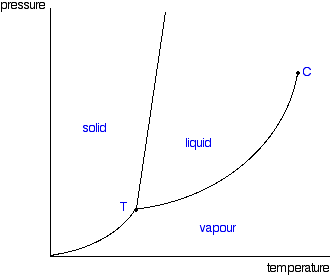
The air transitions from a gas into a liquid as we increase pressure. It so happens 40 meters, or 5 ata, is that pressure (assuming the air is at 20 C or 68 F). This is why diving in cold water is more challenging, because the pressure at the phase change is lower. This is also why warm water diving is generally easier.

See this here phase diagram?

The air transitions from a gas into a liquid as we increase pressure. It so happens 40 meters, or 5 ata, is that pressure (assuming the air is at 20 C or 68 F). This is why diving in cold water is more challenging, because the pressure at the phase change is lower. This is also why warm water diving is generally easier.





