When you fill the tank, then molecules will hit the walls more times per second. Hence the cylinder walls heat up (vibrate). This heat is then dissipated into the environment. Eventually, an equilibrium is achieved. This is the first energy loss.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Making electricity with scuba regulator, has it been done?
- Thread starter Gone for diving
- Start date
Please register or login
Welcome to ScubaBoard, the world's largest scuba diving community. Registration is not required to read the forums, but we encourage you to join. Joining has its benefits and enables you to participate in the discussions.
Benefits of registering include
- Ability to post and comment on topics and discussions.
- A Free photo gallery to share your dive photos with the world.
- You can make this box go away
OMyMyOHellYes
Contributor
Energy is never lost.When you fill the tank, then molecules will hit the walls more times per second. Hence the cylinder walls heat up (vibrate). This heat is then dissipated into the environment. Eventually, an equilibrium is achieved. This is the first energy loss.
Just converted.
OP
Gone for diving
Contributor
When you fill the tank, then molecules will hit the walls more times per second. Hence the cylinder walls heat up (vibrate). This heat is then dissipated into the environment. Eventually, an equilibrium is achieved. This is the first energy loss.
When the heat dissipates,
the pressure in the tank drops,
then I refill it,
So that loss can't be counted,
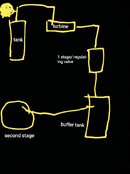
Here's a quick little, very lousy, drawing, I make on my phone,
Just to help visualize,,,
Attachments
So what would the benefit of adding complexity to your breathing gas to run your flashlight and your heated suit?
halocline
Contributor
I think it would be more efficient to have a very small tank to supply air to a hamster running on one of those circular treadmill type things, and have that power your light.
The Ruttmeister
Contributor
Aren't pressure and temperature about the same thing? Molecules hitting the walls? One measures the amount of collisions (amount of stuff) times speed, ie. pressure, the other measures speed only (temp).
For a fixed volume, yes. Boyle's law/ideal gas equation.
Although I'm a little rusty, its over 20 years since I had to learn that stuff.
The difference is that temperature is energy that will be lost out of the system, into the water for example.
I am sure I am, not the first one to think of this.... so that explains why its probably not been done,
Reading your first post about 6 times,
Its starting to make some sense,
Being a mechanic and working on some AC systems, I have some basic knowledge of how this work,
Can you explain in basic terms then,
Where all the bulk of the energy in the dive tank goes, as you breathe it,?
Sure some is moving valve's, noise etc,
temp is the big one, from what I understood...
As the gas leaves the tank, it goes through a series of expansions in volume. In the regulators, in your lungs, in the water. All of that is work done, the gas is pushing other atoms out of the way till the pressure equalizes. In practical terms its all lost to entropy.
The issue you would have is that the regulators are not providing much pressure above ambient, the first is only ~125psi above ambient, the second stage maybe 2-3psi (and maybe not even that much). Plus in air tool terms you are carrying a tiny amount of air.
Air motors are not very efficient, so the practical energy you could extract is going to be pretty marginal. Maybe if you used a triple expansion engine (pistons) you might get a bit better, but that's even less practical.
Compressed air as a source of power is generally only used for safety reasons these days. An air line might be more safe or practical than an electrical line.
Batteries are easier, redundant and more importantly not a potential failure point in your life support.
y
This will freeze your regulator. And you cannot inhale air so cold, it will burn your airways...
I really do not understand why you are ignoring the problem of temperature drop.
There is no energy to be harvested in your compressed air.
High pressure does not increase air's enthalpy.
In some cases (cold water diving) you possibly need external energy to be given to air, for keeping it warm...
After the turbine (adiabatic expansion) the temperature is very low, something as -50 °C, or even lower.Here's a quick little, very lousy, drawing, I make on my phone,
Just to help visualize,,,
This will freeze your regulator. And you cannot inhale air so cold, it will burn your airways...
I really do not understand why you are ignoring the problem of temperature drop.
There is no energy to be harvested in your compressed air.
High pressure does not increase air's enthalpy.
In some cases (cold water diving) you possibly need external energy to be given to air, for keeping it warm...
Pressure does not provide any increase in enthalpy.So, in terms of the kinetic gas theory,
enthalpy is a measure of the (kinetic) energy of all the gas atoms/molecules together?
So, it tells what the absolute maximum amount of work is that a certain amount of (heated?) gas can do?
Oh, you already said "the total energy available"... So probably yes.
Aren't pressure and temperature about the same thing? Molecules hitting the walls? One measures the amount of collisions (amount of stuff) times speed, ie. pressure, the other measures speed only (temp).
1 kg of air at 200 bars and 300 K has substantially the same enthalpy of 1 kg of air at 1 bar and 300 K.
OP
Gone for diving
Contributor
y
After the turbine (adiabatic expansion) the temperature is very low, something as -50 °C, or even lower.
This will freeze your regulator. And you cannot inhale air so cold, it will burn your airways...
I really do not understand why you are ignoring the problem of temperature drop.
There is no energy to be harvested in your compressed air.
High pressure does not increase air's enthalpy.
In some cases (cold water diving) you possibly need external energy to be given to air, for keeping it warm...
I have read alot of your posts and understand you are a very smart guy,
thats one reason I posted my idea, because often it's been tried before, and or the idea fails for other reasons,,,
I was always under the impression the large drops in pressure with a regulator is wasteful if you only need low pressure supply.
Other than storage capacity don't pump higher than needed.
That meant there is energy to gotten in the first stage area..
I am not ignoring the -50* problem, if that's the main and only problem, that can be easily fixed. (We swim in the biggest coldsink ever,
I was not planning on this looking like a conventional regulator there will be a few extra pieces,,,
One day when I have nothing to do (that's highly unlikely)
I will maybe piece a few things together and see what happens....
But until then its probably theory for me,
The Ruttmeister
Contributor
Your biggest issue if you want to test this is available parts.
Existing air motors are generally setup for high CFM air supply, and really high RPM. They are basically turbines. The low air use of a diver (compared to the supply from a shop compressor) means you'll probably not even spin the motor.
I would expect you'd need to build something piston based, plus a basic energy storage device (like a capacitor or battery). As you'll want constant power despite only requiring air intermittently.
You also need to figure out what to do about pressure drop. The first stage is normally putting out ~125psi, the second stage might not function correctly if the power generation drops that pressure too much. Depends on the second stage design.
Building a bench setup to see how much power can be extracted probably isn't too difficult.
Then you would need to do some calculations to figure out how it would perform at depth.
Existing air motors are generally setup for high CFM air supply, and really high RPM. They are basically turbines. The low air use of a diver (compared to the supply from a shop compressor) means you'll probably not even spin the motor.
I would expect you'd need to build something piston based, plus a basic energy storage device (like a capacitor or battery). As you'll want constant power despite only requiring air intermittently.
You also need to figure out what to do about pressure drop. The first stage is normally putting out ~125psi, the second stage might not function correctly if the power generation drops that pressure too much. Depends on the second stage design.
Building a bench setup to see how much power can be extracted probably isn't too difficult.
Then you would need to do some calculations to figure out how it would perform at depth.
Similar threads
- Replies
- 0
- Views
- 312
- Replies
- 73
- Views
- 4,737
- Replies
- 33
- Views
- 2,005